Recently, a number of plaintiff verdicts and group settlements have marked a promising trend of holding large pharmaceutical companies accountable for the harm that their pelvic mesh devices cause. While this is undeniably good news, these same companies continue to turn a blind eye to the dangers of their nearly identical hernia mesh products.
Pelvic mesh is a net-like sheet that is surgically implanted in patients with a weakened bladder neck, urethra, or vaginal wall. It is used in individuals with pelvic floor conditions such as stress urinary incontinence (SUI) and pelvic organ prolapse (POP), both of which are commonly seen in women after childbirth. There are about 200,000 inpatient surgical procedures done in the U.S. annually on individuals with POP.
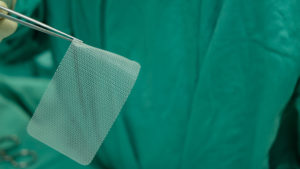
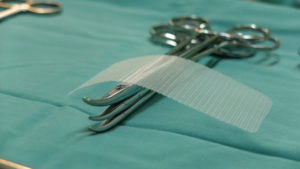
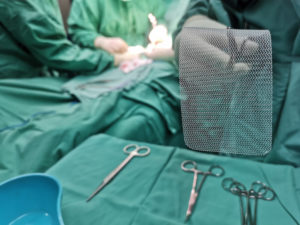
Hernia mesh is used even more commonly. Approximately one million hernia repairs are done each year in the United States, over 90 percent of which use surgical mesh. A hernia is when an organ bursts through a weakened cavity wall. The most prevalent type of hernia involves an intestine protruding through the abdominal wall, commonly known as an abdominal or ventral hernia. Hernia mesh holds the weakened wall in place so that the tissue can grow into the mesh’s pores and form a stronger wall of support.
Tens of thousands of individuals have suffered from detrimental health consequences caused by surgical mesh. Mesh erosion, infections, bleeding, pain during sex, organ perforations, and urinary problems can result from pelvic mesh. Hernia mesh patients see many of the same complications, often resulting from a dislodging or contraction of the mesh product. The contraction of the mesh can cause severe and chronic pain that may not be cured until the mesh product is removed. Hernia mesh products are also susceptible to increased inflammation, infection, poor or lack of healing, buildup of fluid or warmth at the incision sign, flu-like symptoms including vomiting, chills, and fever, chronic pain or discomfort, scarring, and abscess or fistula formation.
Pelvic mesh plaintiffs have been seeing some big wins lately: on April 10, a Superior Court panel voted unanimously to uphold a $13.5 million verdict against Johnson & Johnson (J&J) after a plaintiff was injured by their pelvic mesh device implantation. Just two weeks later, on April 24, a Philadelphia court jury awarded another plaintiff $120 million in damages after her pelvic mesh led to chronic pain, incontinence, and lack of consortium. Both of these successes came after a New York Times article revealed that seven device manufacturers are paying nearly $8 billion to settle more than 100,000 pelvic mesh cases.
Despite the undeniable similarities between pelvic and hernia mesh and the FDA’s nationwide ban on pelvic mesh sales due to safety concerns, most hernia mesh products remain available to consumers. While there have been FDA-issued recalls for some hernia mesh products, mesh implantation continues to be the standard for hernia treatment. On a more positive note, however, big pharma manufacturers including J&J, Gore, Atrium, and C.R. Bard will all be facing new litigation and/or bellwether trials for hernia mesh cases in 2019. Hopefully, upcoming verdicts succeed in holding these manufacturers responsible for the pain and suffering hernia mesh causes.
If you or a loved one has experienced complications after a hernia mesh implant, call Pogust Millrood toll free at 888-348-6787 or direct to our Pennsylvania office at 610-941-4204 to see if you are entitled to seek damages.

A Pennsylvania native, Mike Daly has spent most of his life in the greater Philadelphia area. A proud graduate of Penn State and the Villanova School of Law, he's currently a partner at Pogust Millrood, where he represents individuals throughout the country in class action and mass tort claims involving consumer fraud, environmental harm, defective medical devices, and harmful pharmaceuticals.









Comments for this article are closed.