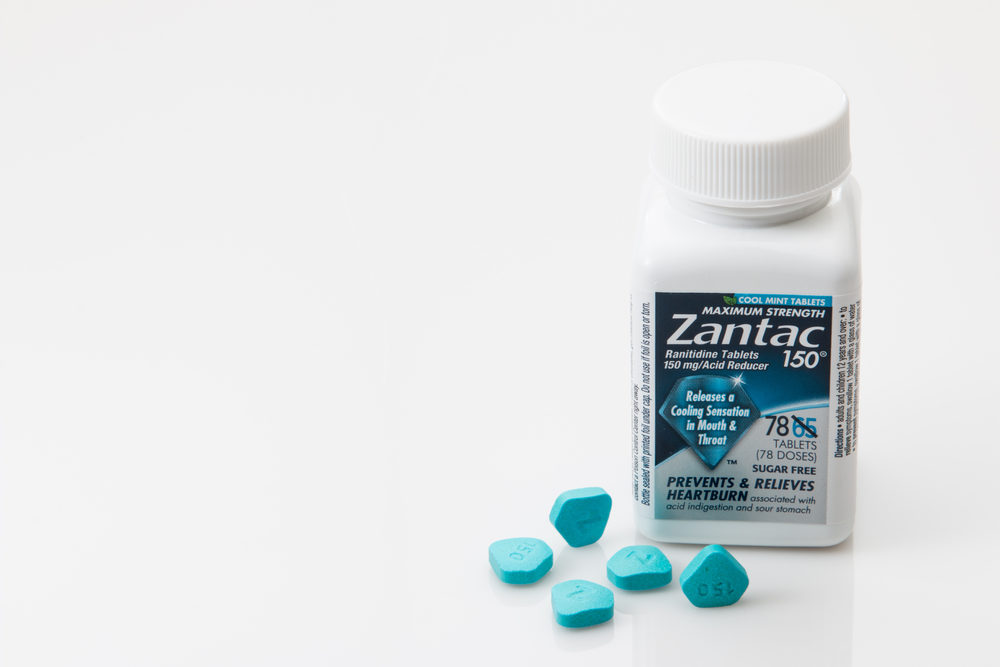
Ranitidine, also known by its brand name Zantac, belongs to a class of drugs known as H2 (or histamine-2) blockers. Over the counter (OTC) ranitidine (Zantac) is commonly used to relieve and prevent heartburn while prescription strengths are used to treat and prevent more serious ulcers in the stomach and intestines.

In the summer of 2019, laboratory tests began showing low levels of a potentially cancer-causing contaminant known as N-Nitrosodimethylamine (NDMA) in some ranitidine (Zantac) medicines. Shortly thereafter, several drug makers issued voluntary recalls of ranitidine (Zantac) medicines and the U.S. Food and Drug Administration (FDA) alerted health care professionals and patients of the potential cancer risk.<
On April 1, 2020, the U.S. Food and Drug Administration (FDA) requested that manufacturers immediately withdraw all prescription and OTC ranitidine medications from the market after further testing concluded that a potentially unsafe amount of NDMA can build up in the drug when stored for long periods or in the presence of heat.
The FDA recommended consumers stop taking and dispose of any over the counter ranitidine (Zantac) tablets or liquid that they currently have at home. Patients who are taking prescription ranitidine should speak with their health care professional about other treatment options before stopping the medicine. There are many other drugs that can be used to treat the same conditions ranitidine (Zantac) has been used to treat.
There are a variety of companies that produced and marketed ranitidine in the United States. Lawsuits are being filed on behalf of people who have injuries such as liver, bladder and other types of cancer, which they attribute to NDMA in Zantac. The filings claim that manufacturers, sellers and distributors knowingly put consumers at risk and were responsible for hiding the dangers of Zantac from its consumers.